Abstract
Background:
Lipocalin-2 (LCN2) is widely expressed in the organism with pleiotropic roles. In particular, its overexpression correlates with tissue stress conditions including inflammation, metabolic disorders, chronic diseases and cancer.
Objectives:
To assess the effects of systemic LCN2 overexpression on adipose tissue and glucose metabolism.
Subjects:
Eighteen-month-old transgenic mice with systemic LCN2 overexpression (LCN2-Tg) and age/sex-matched wild-type mice.
Methods:
Metabolic cages; histology and real-time PCR analysis; glucose and insulin tolerance tests; ELISA; flow cytometry; microPET and serum analysis.
Results:
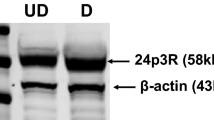
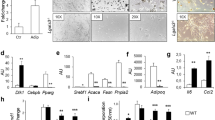
LCN2-Tg mice were smaller compared to controls but they ate (P = 0.0156) and drank (P = 0.0057) more and displayed a higher amount of visceral adipose tissue. Furthermore, LCN2-Tg mice with body weight ≥20 g showed adipocytes with a higher cell area (P < 0.0001) and altered expression of genes involved in adipocyte differentiation and inflammation. In particular, mRNA levels of adipocyte-derived Pparg (P ≤ 0.0001), Srebf1 (P < 0.0001), Fabp4 (P = 0.056), Tnfa (P = 0.0391), Il6 (P = 0.0198), and Lep (P = 0.0003) were all increased. Furthermore, LCN2-Tg mice displayed a decreased amount of basal serum insulin (P = 0.0122) and a statistically significant impaired glucose tolerance and insulin sensitivity consistent with Slc2a2 mRNA (P ≤ 0.0001) downregulated expression. On the other hand, Insr mRNA (P ≤ 0.0001) was upregulated and correlated with microPET analysis that demonstrated a trend in reduced whole-body glucose consumption and MRGlu in the muscles and a significantly reduced MRGlu in brown adipose tissue (P = 0.0247). Nevertheless, an almost nine-fold acceleration of hexokinase activity was observed in the LCN2-Tg mice liver compared to controls (P = 0.0027). Moreover, AST and ALT were increased (P = 0.0421 and P = 0.0403, respectively), which indicated liver involvement also demonstrated by histological staining.
Conclusions:
We show that LCN2 profoundly impacts adipose tissue size and function and glucose metabolism, suggesting that LCN2 should be considered as a risk factor in ageing for metabolic disorders leading to obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Meheus LA, Fransen LM, Raymackers JG, Blockx HA, Van Beeumen JJ, Van Bun SM, et al. Identification by microsequencing of lipopolysaccharide-induced proteins secreted by mouse macrophages. J Immunol. 1993;151:1535–47.
Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;14:1–14.
Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21.
Liu Q, Ryon J, Nilsen-Hamilton M. Uterocalin: a mouse acute phase protein expressed in the uterus around birth. Mol Reprod Dev. 1997;46:507–14.
Cermelli S, Zerega B, Carlevaro M, Gentili C, Thorp B, Farquharson C, et al. Extracellular fatty acid binding protein (Ex-FABP) modulation by inflammatory agents: ‘physiological’ acute phase response in endochondral bone formation. Eur J Cell Biol. 2000;79:155–64.
Costa D, Lazzarini E, Canciani B, Giuliani A, Spanò R, Marozzi K, et al. Altered bone development and turnover in transgenic mice over-expressing Lipocalin-2 in bone. J Cell Physiol. 2013;228:2210–21.
Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. J Biol Chem. 1995;270:22565–70.
Ariza X, Graupera I, Coll M, Sola E, Barreto R, Garcia E, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65:57–65.
Costa D, Principi E, Lazzarini E, Descalzi F, Cancedda R, Castagnola P, et al. LCN2 overexpression in bone enhances the hematopoietic compartment via modulation of the bone marrow microenvironment. J Cell Physiol. 2017;232:1–24.
Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–76.
Bartsch S, Tschesche H. Cloning and expression of human neutrophil lipocalin cDNA derived from bone marrow and ovarian cancer cells. FEBS Lett. 1995;357:255–9.
Furutani M, Arii S, Mizumoto M, Kato M, Imamura M. Identification of a neutrophil gelatinase-associated lipocalin mRNA in human pancreatic cancers using a modified signal sequence trap method. Cancer Lett. 1998;122:209–14.
Stoesz SP, Friedl A, Haag JD, Lindstrom MJ, Clark GM, Gould MN. Heterogeneous expression of the lipocalin NGAL in primary breast cancers. Int J Cancer. 1998;79:565–72.
Auguet T, Quintero Y, Terra X, Martinez S, Lucas A, Pellitero S, et al. Upregulation of lipocalin 2 in adipose tissues of severely obese women: positive relationship with proinflammatory cytokines. Obesity (Silver Spring). 2011;19:2295–300.
Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–55.
Moreno-Navarrete JM, Manco M, Ibáñez J, García-Fuentes E, Ortega F, Gorostiaga E, et al. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int J Obes (Lond). 2010;34:240–9.
Jang Y, Lee JH, Wang Y, Sweeney G. Emerging clinical and experimental evidence for the role of lipocalin-2 in metabolic syndrome. Clin Exp Pharmacol Physiol. 2012;39:194–9.
Tan BK, Adya R, Shan X, Syed F, Lewandowski KC, O’hare JP, et al. Ex vivo and in vivo regulation of lipocalin-2, a novel adipokine, by Insulin. Diabetes Care. 2009;32:129–31.
Jun LS, Siddall CP, Rosen ED. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. Am J Physiol Endocrinol Metab. 2011;301:E825–35.
Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543:385–90.
Costa D, Biticchi R, Negrini S, Tasso R, Cancedda R, Descalzi F, et al. Lipocalin-2 controls the expression of SDF-1 and the number of responsive cells in bone. Cytokine. 2010;51:47–52.
Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–34.
Marini C, Bianchi G, Buschiazzo A, Ravera S, Martella R, Bottoni G, et al. Divergent targets of glycolysis and oxidative phosphorylation result in additive effects of metformin and starvation in colon and breast cancer. Sci Rep. 2016;6:19569.
Marini C, Salani B, Massollo M, Amaro A, Esposito AI, Orengo AM, et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle. 2013;12:3490–9.
Bianchi G, Martella R, Ravera S, Marini C, Capitanio S, Orengo A, et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget. 2015;6:11806–19.
Garbarino S, Vivaldi V, Delbary F, Caviglia G, Piana M, Marini C, et al. A new compartmental method for the analysis of liver FDG kinetics in small animal models. EJNMMI Res. 2015;5:35.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. generalizations. J Cereb Blood Flow Metab. 1985;5:584–90.
Amri EZ, Bertrand B, Ailhaud G, Grimaldi P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res. 1991;32:1449–56.
Tso AW, Xu A, Chow WS, Lam KS. Adipose tissue and the metabolic syndrome: focusing on adiponectin and several novel adipokines. Biomark Med. 2008;2:239–52.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808.
Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80.
Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56.
Brennan AM, Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–27.
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks [see comments]. Science. 1995;269:546–9.
Steinberg GR. Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle. AJP Endocrinol Metab. 2003;286:57E–63.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
Wang Y, Lam KSL, Kraegen EW, Sweeney G, Zhang J, Tso AWK, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41.
Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–40.
Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–69.
Kehrer JP. Lipocalin-2: pro- or anti-apoptotic? Cell Biol Toxicol. 2010;26:83–9.
Leng X, Lin H, Ding T, Wang Y, Wu Y, Klumpp S, et al. Lipocalin 2 is required for BCR-ABL-induced tumorigenesis. Oncogene. 2008;27:6110–9.
Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–85.
Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA, et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 2016;24:210–22.
Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26:677–80.
Acknowledgements
We wish to thank Katia Cortese (University of Genoa, Italy) for the lipid droplets analysis. This work was partially supported by funds from Italian Space Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Principi, E., Buschiazzo, A., Papait, A. et al. Anthropometric and glucometabolic changes in an aged mouse model of lipocalin-2 overexpression. Int J Obes 43, 189–201 (2019). https://doi.org/10.1038/s41366-018-0171-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0171-5